In case you intend to fill out form gmp pdf, you don't need to download and install any kind of programs - just try using our online PDF editor. The tool is constantly upgraded by our staff, acquiring powerful functions and turning out to be much more convenient. Here is what you will want to do to get going:
Step 1: First, access the editor by pressing the "Get Form Button" in the top section of this webpage.
Step 2: After you launch the PDF editor, you will find the form prepared to be completed. Aside from filling in various blanks, you may as well perform various other actions with the PDF, particularly putting on any textual content, editing the initial textual content, inserting images, affixing your signature to the PDF, and a lot more.
As for the blank fields of this precise form, here's what you want to do:
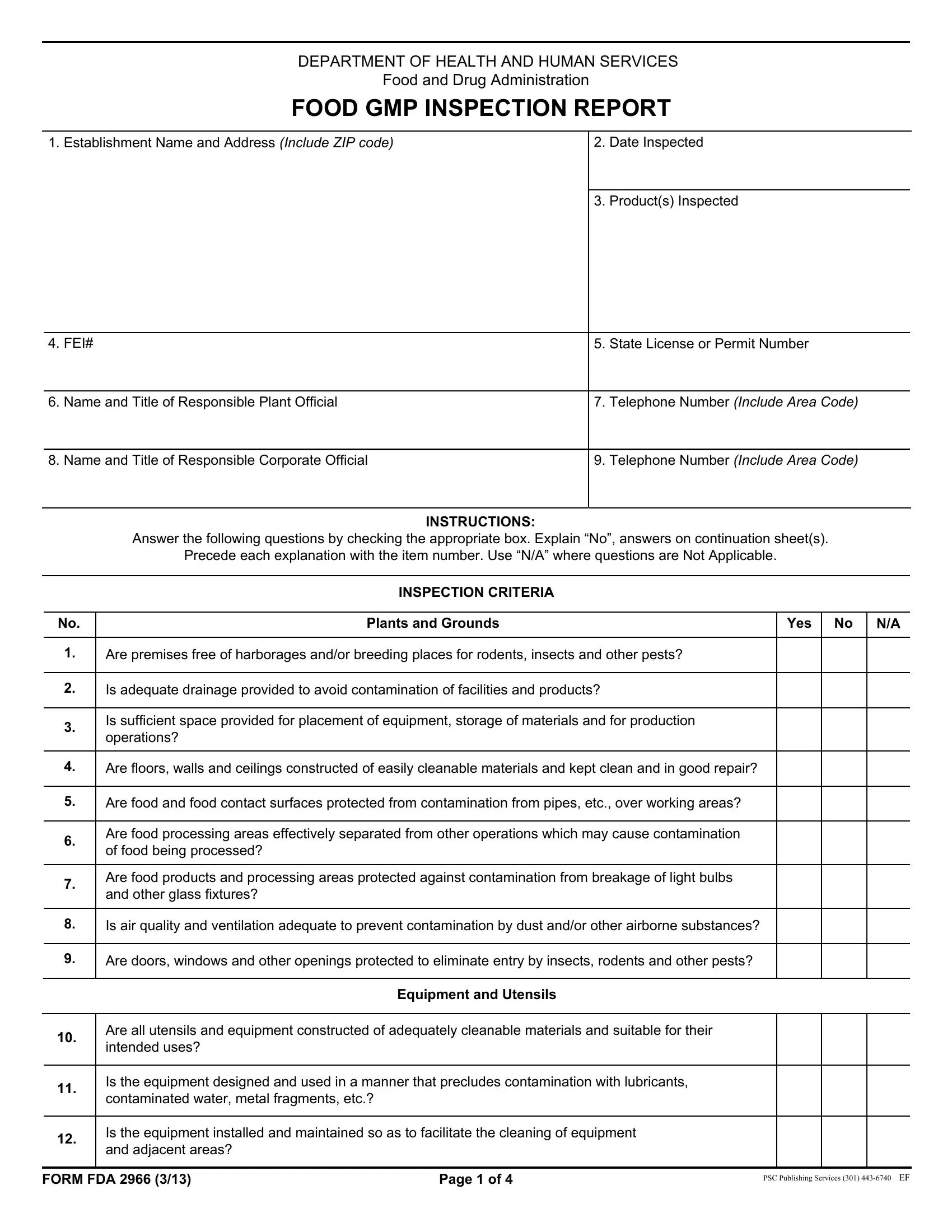
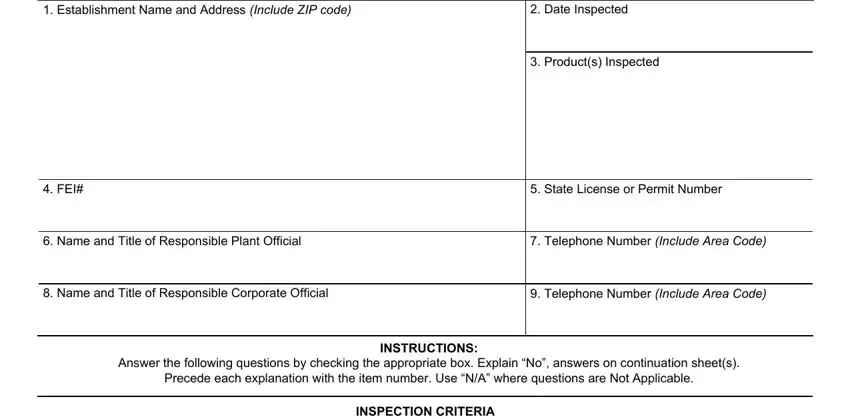
1. You have to fill out the form gmp pdf properly, so be careful while filling in the segments comprising all these fields:
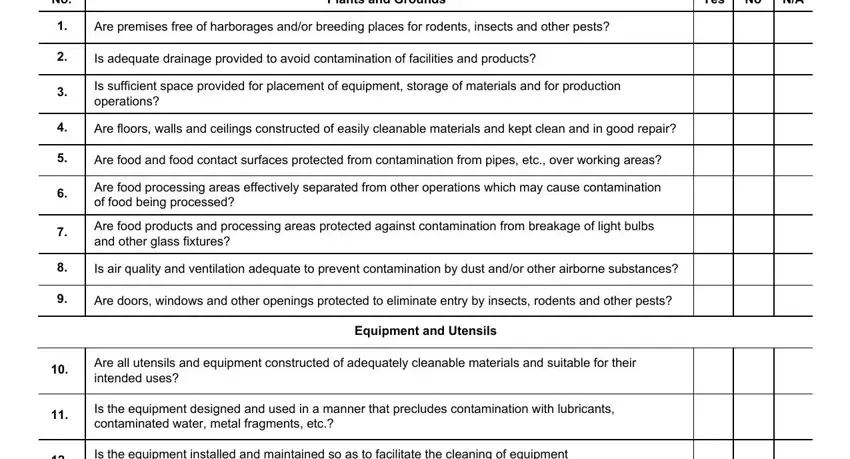
2. Right after the previous part is done, go on to enter the relevant information in these - Plants and Grounds, Yes, Are premises free of harborages, Is adequate drainage provided to, Is sufficient space provided for, Are floors walls and ceilings, Are food and food contact surfaces, Are food processing areas, Are food products and processing, Is air quality and ventilation, Are doors windows and other, Equipment and Utensils, Are all utensils and equipment, Is the equipment designed and used, and Is the equipment installed and.
3. The following section will be about Sanitary Facilities and Controls, Yes, Is the water supply adequate in, Are the water temperatures and, Is the sewage disposal system, Is the plumbing adequately sized, Are adequate toilet rooms provided, Are adequate handwashing andor, Is all refuse properly stored and, Is the facility kept clean and in, Sanitary Operations, Is cleaning of facilities and, Are detergents sanitizers, and Are cleaning compounds and - fill in all of these empty form fields.
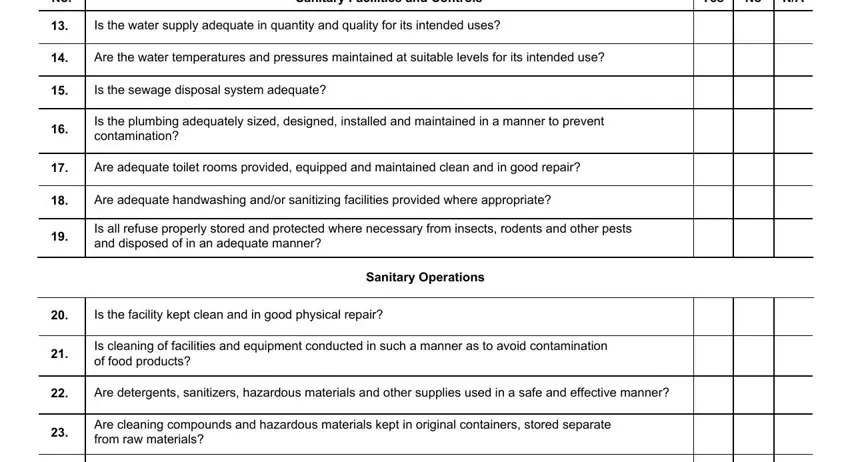
4. Your next part will require your input in the subsequent parts: Are the processing areas, Are insecticides and rodenticides, Are all utensils and equipment, Are single service articles stored, Are utensils and portable, Processes and Controls, Is responsibility for overall, Are raw materials and ingredients, Is ice where used manufactured, Is food processing conducted in a, and Are chemical microbiological or. It is important to type in all requested information to move forward.
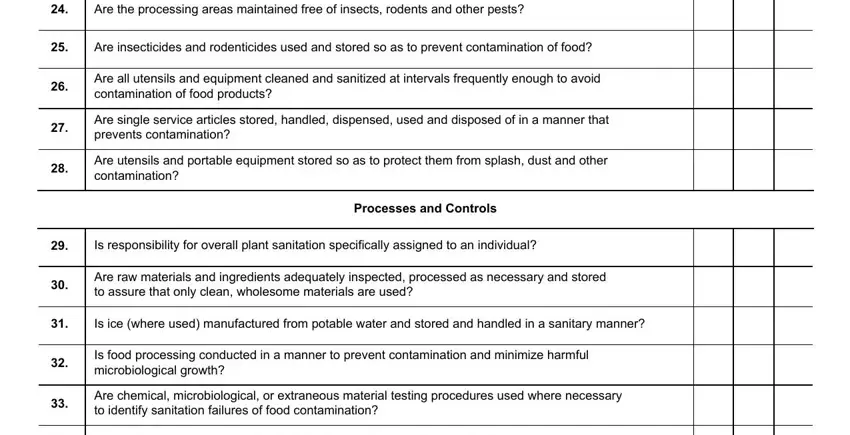
Always be really attentive while filling in Are raw materials and ingredients and Processes and Controls, because this is where many people make errors.
5. The very last point to complete this form is essential. You must fill in the required blank fields, particularly Are packaging processes and, Are only approved food andor color, FORM FDA, and Page of, prior to submitting. Neglecting to do this may give you an unfinished and possibly unacceptable form!

Step 3: Prior to obtaining the next stage, ensure that all blanks were filled in correctly. When you establish that it's correct, click on “Done." Try a 7-day free trial subscription with us and acquire direct access to form gmp pdf - download or edit from your personal account. At FormsPal, we aim to be sure that all of your details are maintained secure.