By using the online editor for PDFs by FormsPal, you are able to fill in or edit variance c device download right here. Our editor is constantly developing to deliver the best user experience achievable, and that's thanks to our dedication to constant development and listening closely to feedback from users. All it takes is just a few easy steps:
Step 1: First of all, access the tool by clicking the "Get Form Button" in the top section of this webpage.
Step 2: After you open the tool, you will find the document ready to be completed. Aside from filling out various blanks, you could also perform other sorts of things with the file, namely adding any text, changing the original text, adding images, affixing your signature to the PDF, and a lot more.
This PDF form will require particular information to be entered, therefore you should take your time to enter precisely what is expected:
1. When filling out the variance c device download, ensure to complete all of the important fields within its relevant area. This will help facilitate the process, allowing your information to be handled fast and properly.
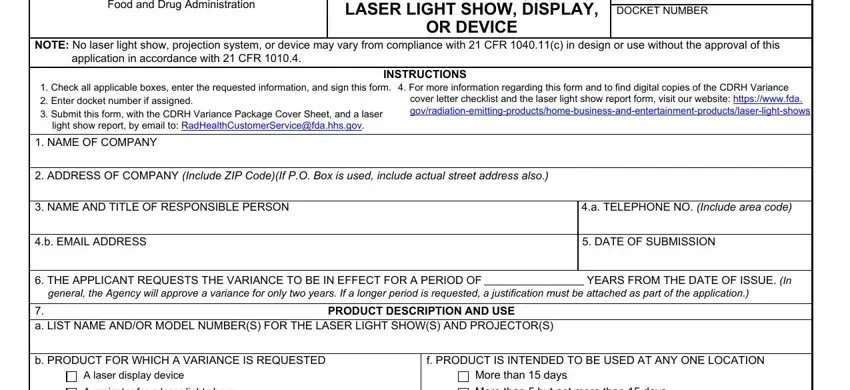
2. Just after filling in the last part, go on to the subsequent stage and complete the essential particulars in these fields - A projector for a laser light show, A laser light show, Other Specify, More than but not more than days, Less than days, g TOUR IS INTENDED TO RUN FOR, PROJECTORS ARE INTENDED FOR SALE, d PRODUCT IS INTENDED FOR USE IN A, Planetarium or other dome, Theater, More than months, months, Less than one month, Not applicable Not a tour, and Other Specify.
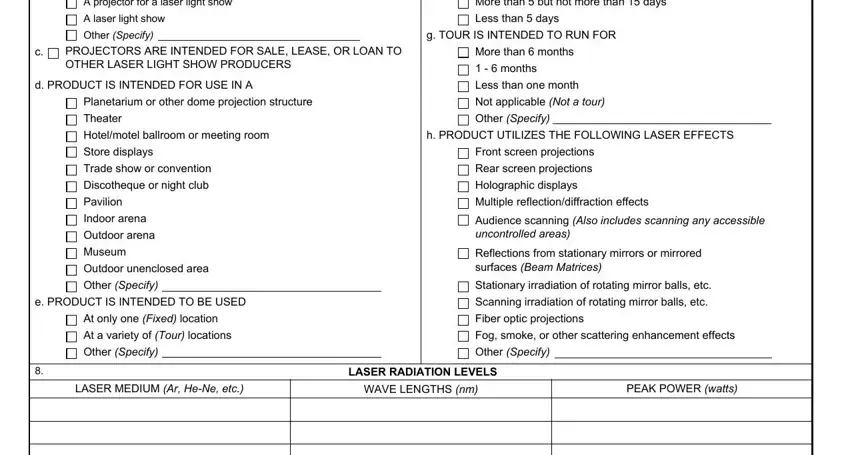
3. Completing IF ANY LASER RADIATION IS PULSED, REASON FOR REQUESTING VARIANCE, Compliance with the limits of CFR, Other or additional explanation, FORM FDA, PREVIOUS EDITION IS OBSOLETE, PAGE OF PAGES, and PSC Publishing Services is essential for the next step, make sure to fill them out in their entirety. Don't miss any details!

4. This next section requires some additional information. Ensure you complete all the necessary fields - MANNER IN WHICH IT IS PROPOSED TO, It is proposed to deviate from the, It is proposed to deviate from the, ADVANTAGES TO BE DERIVED FROM, Laser light shows and displays are, Other or additional advantages, EXPLAIN THE ALTERNATE MEANS OF, any boxes not checked using, All laser products systems shows, Effects not specifically indicated, and Scanning projection or reflection - to proceed further in your process!

5. Since you reach the end of the form, there are actually a few more requirements that have to be met. Specifically, Laser radiation levels in excess, Any product which relies on, All laser light shows shall be, Be an employee of the variance, Be located where all beam paths, Immediately terminate the, by any air traffic control, The maximum laser projector output, The projection system ie the, Laser projectors will not be, In addition to the requirements of, and The requirements of CFR a and should be filled out.

A lot of people generally make errors while filling in Laser radiation levels in excess in this part. Be sure you review whatever you enter right here.
Step 3: Make sure the details are right and click on "Done" to complete the task. Acquire the variance c device download as soon as you register online for a free trial. Instantly use the form in your personal cabinet, with any edits and changes being conveniently kept! We don't share the information that you use while dealing with forms at our website.