Completion of Form FDA 3356 is required under 21 CFR Part 1271, 207.20 and 807.20 for all establishments engaged in the recovery, processing, storage, labeling, packaging, or distribution of any HCT/P, or the screening or testing of a cell or tissue donor. After we receive your form, we will update our records and send a validated form to the reporting official.
PART I. ESTABLISHMENT INFORMATION
NOTE: You are required to register and list your HCT/Ps by submitting this form if you recover, pro- cess, store, label, package, or distribute any HCT/P, or screen or test the HCT/P donor unless one of the following exceptions applies. You are not required to submit this form if:
a.You use HCT/Ps solely for nonclinical scientific or educational purposes,
b.You remove and then implant HCT/Ps solely for autologous use during the same surgical procedure,
c.You are a carrier who accepts, receives, carries, or delivers HCT/Ps in the usual course of business as a carrier,
d.You only receive or store HCT/Ps solely for implantation, transplantation, infusion, or transfer within your facility,
e.You only recover reproductive cells or tissue and immediately transfer them into a sexually inti- mate partner of the cell or tissue donor, or
f.You are an individual person who works under contract, agreement, or other arrangement with or for a registered establishment and only recover and send HCT/Ps to the registered establishment.
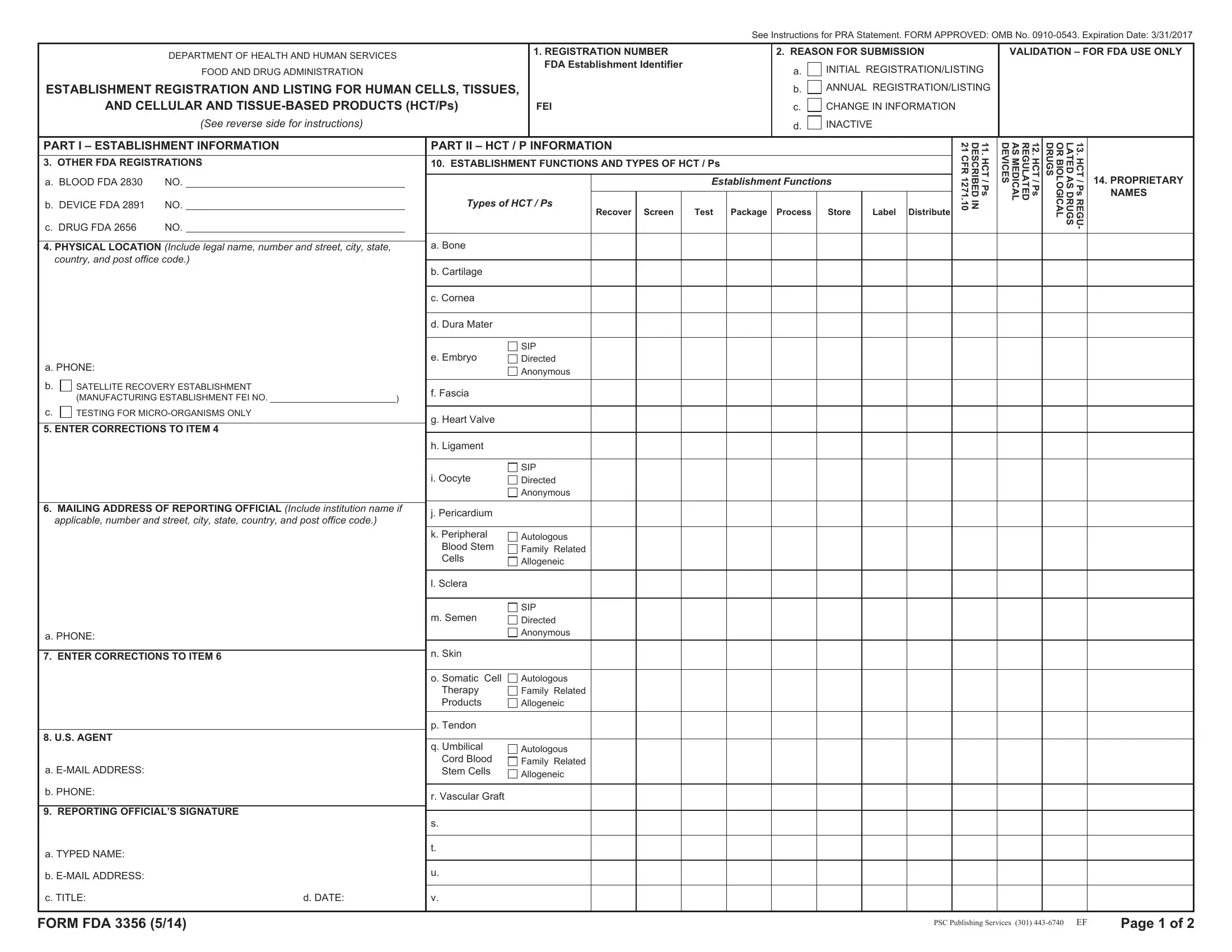
Item 3. OTHER FDA REGISTRATIONS – Provide the registration number if your establishment is already registered with FDA as a Blood, Medical Device or Drug establishment. Your establishment will not be given a new registration number, and you are not required to fill in items 4 to 8 of Part I. Item 9 must be filled out and signed on all forms. If you choose not to complete Items 4 to 8 of Part I, you still must complete and sign Item 9. Then proceed to Part II and provide product information.
Item 4. PHYSICAL LOCATION – Provide the legal name, street address including the postal code of the actual location and
a. Telephone number.
b.Indicate (with an X) if you are a satellite recovery establishment that supports recovery personnel in the field by providing temporary storage for recovered HCT/Ps for shipment to your parent manufacturing establishment, but do not perform any other activities or manufacturing steps. Pro- vide the FEI NO. of your parent manufacturing establishment.
c.Indicate (with an X) if you are an establishment that performs testing of HCT/Ps for micro-organ- isms if that is the only HCT/P processing function that you perform.
Item 6. MAILING ADDRESS OF THE REPORTING OFFICIAL – Provide the reporting offcial’s mailing address including the postal code if it is different from the actual location of the establishment.
Items 8. U.S. AGENT – Non-U.S. establishments only: Provide your U.S. agent name, institution name if applicable, street address, e-mail address, and telephone number. United States agent means a person residing or maintaining a place of business in the United States whom a foreign establishment designates as its agent.
Item 9. REPORTING OFFICIAL’S SIGNATURE – The reporting official as listed in Item 6 is the person appointed by the owner or operator to register the form and answer all the correspondence and inquiries relative thereto. The dated signature by the reporting official affirms that all information contained on the form is true and accurate, to the best of his or her knowledge.
PART II. HCT/P INFORMATION (If item 2.c is checked, only indicate the information being changed.)
Item 10. ESTABLISHMENT FUNCTIONS AND TYPES OF HCT/Ps – Indicate (with an X) the activity (ies) performed by the registered establishment in conjunction with the type of HCT/P that the registered establishment manufactures. Test and screen refer to the donor, not the HCT/P. For reproductive HCT/Ps, indicate whether the HCT/Ps are from sexually intimate partners (SIP), directed, or anonymous. For hematopoietic stem/progenitor cells and somatic cells, indicate whether the HCT/ Ps are autologous, family related, or allogeneic. Family related means allogeneic use in a first-degree or second-degree relative.
Item 11. LISTING FOR HCT/Ps DESCRIBED IN 21 CFR 1271.10 – To list HCT/Ps that are described in 21 CFR 1271.10 (a) indicate (with an X) each HCT/P that fulfills all of the following criteria:
a.The HCT/P is minimally manipulated,
b.The HCT/P is intended for homologous use only, as reflected by the labeling, advertising, or other indications of the manufacturer’s objective intent,
c.The manufacture of the HCT/P does not involve the combination of the cell or tissue component with a drug or a device, except for a sterilizing, preserving, or storage agent, if the addition of the agent does not raise new clinical safety concerns with respect to the HCT/P, and either
d.The HCT/P does not have a systemic effect and is not dependent upon the metabolic activity of living cells for its primary function; or the HCT/P has a systemic effect or is dependent upon the metabolic activity of living cells for its primary function, and (i) is for autologous use, (ii) is for allogeneic use in a first-degree or second-degree blood relative, or (iii) is for reproductive use.
If your HCT/P type is not preprinted on the form, list it on lines s-v.
Item 12. HCT/P LISTING FOR MEDICAL DEVICES – Indicate (with an X) each HCT/P that is regulated as a medical device under the Federal Food, Drug, and Cosmetic Act.
Item 13. HCT/P LISTING FOR DRUGS OR BIOLOGICAL DRUGS – Indicate (with an X) each HCT/ P that is regulated as a drug or biological drug under the Federal Food, Drug, and Cosmetic Act and/ or section 351 of the Public Health Service Act.
NOTE: For items 11, 12, and 13 indicate changes to HCT/P listing such as discontinuance (indicate with a D), or resumption (indicate with an R) of a HCT/P into commercial distribution in June and December or at the time the change occurs as directed under 21 CFR Part 1271.21. Dates of HCT/P discontinuance/resumption should be provided on an additional page.
Item 14. PROPRIETARY NAMES – Indicate any applicable proprietary names used for the HCT/Ps listed, such as a trademark.
NOTE: If necessary, add an additional page to complete Items 11, 12, 13, or 14.