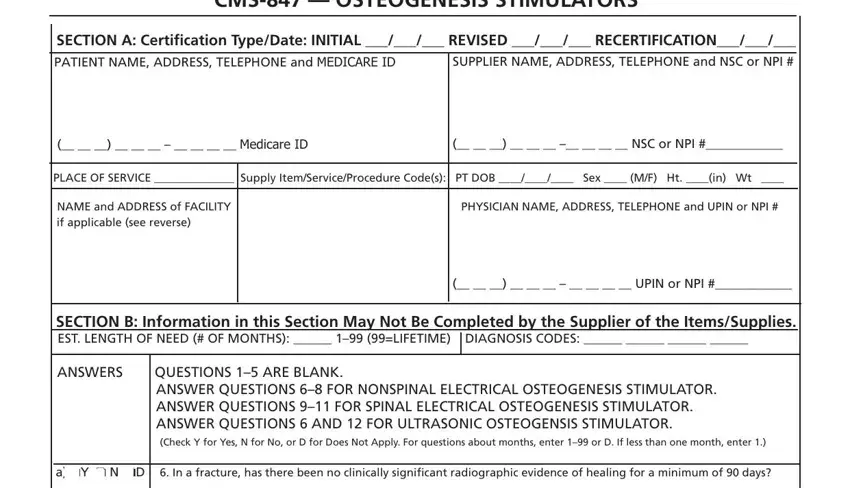
SECTION A: |
(May be completed by the supplier) |
CERTIFICATION |
If this is an initial certification for this patient, indicate this by placing date (MM/DD/YY) needed initially in the space TYPE/ |
DATE: |
marked “INITIAL.” If this is a revised certification (to be completed when the physician changes the order, based on the |
|
patient’s changing clinical needs), indicate the initial date needed in the space marked “INITIAL,” and indicate the |
|
recertification date in the space marked “REVISED.” If this is a recertification, indicate the initial date needed in the |
|
space marked “INITIAL,” and indicate the recertification date in the space marked “RECERTIFICATION.” Whether |
|
submitting a REVISED or a RECERTIFIED CMN, be sure to always furnish the INITIAL date as well as the REVISED or |
|
RECERTIFICATION date. |
PATIENT |
Indicate the patient’s name, permanent legal address, telephone number and his/her Medicare ID as it appears on his/her |
INFORMATION: |
Medicare card and on the claim form. |
SUPPLIER |
Indicate the name of your company (supplier name), address and telephone number along with the Medicare Supplier |
INFORMATION: |
Number assigned to you by the National Supplier Clearinghouse (NSC) or applicable National Provider Identifier (NPI). If |
|
using the NPI Number, indicate this by using the qualifier XX followed by the 10-digit number. If using a legacy number, |
|
e.g. NSC number, use the qualifier 1C followed by the 10-digit number. (For example. 1Cxxxxxxxxxx) |
PLACE OF SERVICE: |
Indicate the place in which the item is being used, i.e., patient’s home is 12, skilled nursing facility (SNF) is 31, End |
|
Stage Renal Disease (ESRD) facility is 65, etc. Refer to the DMERC supplier manual for a complete list. |
FACILITY NAME: |
If the place of service is a facility, indicate the name and complete address of the facility. |
SUPPLY ITEM/SERVICE |
List all procedure codes for items ordered. Procedure codes that do not require certification should not be listed |
PROCEDURE CODE(S): |
on the CMN. |
PATIENT DOB, HEIGHT, |
Indicate patient’s date of birth (MM/DD/YY) and sex (male or female); height in inches and weight in pounds, if requested. |
WEIGHT AND SEX: |
|
PHYSICIAN NAME, |
Indicate the PHYSICIAN’S name and complete mailing address. |
ADDRESS: |
|
PHYSICIAN |
Accurately indicate the treating physician’s Unique Physician Identification Number (UPIN) or applicable National |
INFORMATION: |
Provider Identifier (NPI). If using the NPI Number, indicate this by using the qualifier XX followed by the 10-digit number. |
|
If using UPIN number, use the qualifier 1G followed by the 6-digit number. (For example. 1Gxxxxxx) |
PHYSICIAN’S |
Indicate the telephone number where the physician can be contacted (preferably where records would be accessible |
TELEPHONE NO: |
pertaining to this patient) if more information is needed. |
SECTION B: |
(May not be completed by the supplier. While this section may be completed by a non-physician clinician, or a |
|
Physician employee, it must be reviewed, and the CMN signed (in Section D) by the treating practitioner.) |
EST. LENGTH OF NEED: |
Indicate the estimated length of need (the length of time the physician expects the patient to require use of the ordered |
|
item) by filling in the appropriate number of months. If the patient will require the item for the duration of his/her life, |
|
then enter “99”. |
DIAGNOSIS CODES: |
In the first space, list the diagnosis code that represents the primary reason for ordering this item. List any additional |
|
diagnosis codes that would further describe the medical need for the item (up to 4 codes). |
QUESTION SECTION: |
This section is used to gather clinical information to help Medicare determine the medical necessity for the item(s) |
|
being ordered. Answer each question which applies to the items ordered, checking “Y” for yes, “N” for no, or “D” for |
|
does not apply. |
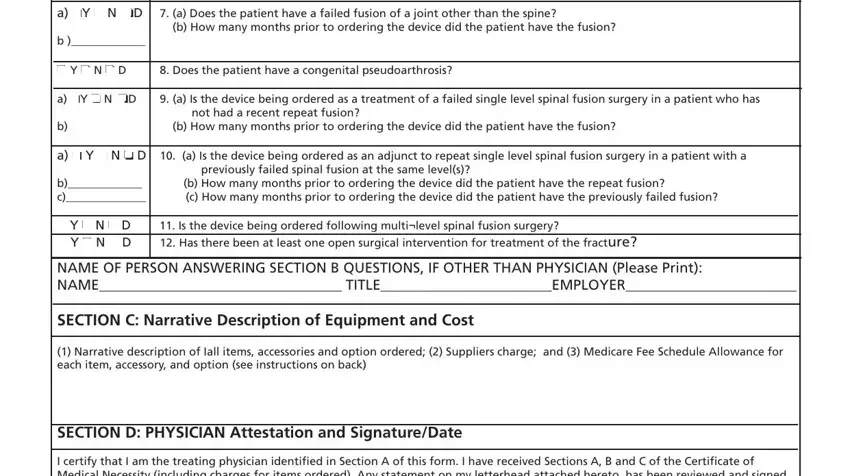
NAME OF PERSON |
If a clinical professional other than the treating physician (e.g., home health nurse, physical therapist, dietician) or a |
ANSWERING SECTION B |
physician employee answers the questions of Section B, he/she must print his/her name, give his/her professional title |
QUESTIONS: |
and the name of his/her employer where indicated. If the physician is answering the questions, this space may be |
|
left blank. |
SECTION C: |
(To be completed by the supplier) |
NARRATIVE |
Supplier gives (1) a narrative description of the item(s) ordered, as well as all options, accessories, supplies and drugs; |
DESCRIPTION OF |
(2) the supplier’s charge for each item(s), options, accessories, supplies and drugs; and (3) the Medicare fee schedule |
EQUIPMENT & COST: |
allowance for each item(s), options, accessories, supplies and drugs, if applicable. |
SECTION D: |
(To be completed by the physician) |
PHYSICIAN |
The physician’s signature certifies (1) the CMN which he/she is reviewing includes Sections A, B, C and D; (2) the |
ATTESTATION: |
answers in Section B are correct; and (3) the self-identifying information in Section A is correct. |
PHYSICIAN SIGNATURE |
After completion and/or review by the physician of Sections A, B and C, the physician’s must sign and date the CMN in |
AND DATE: |
Section D, verifying the Attestation appearing in this Section. The physician’s signature also certifies the items ordered |
|
are medically necessary for this patient. |
According to the Paperwork Reduction Act of 1995, no persons are required to respond to a collection of information unless it displays a valid OMB control number. The valid OMB control number for this information collection is 0938-0679. The time required to complete this information collection is estimated to average 12 minutes per response, including the time to review instructions, search existing resources, gather the data needed, and complete and review the information collection. If you have any comments concerning the accuracy of the time estimate or suggestions for improving this form, please write to: CMS, Attn: PRA Reports Clearance Officer, 7500 Security Blvd. Baltimore, Maryland 21244.
DO NOT SUBMIT CLAIMS TO THIS ADDRESS. Please see http://www.medicare.gov/ for information on claim filing.