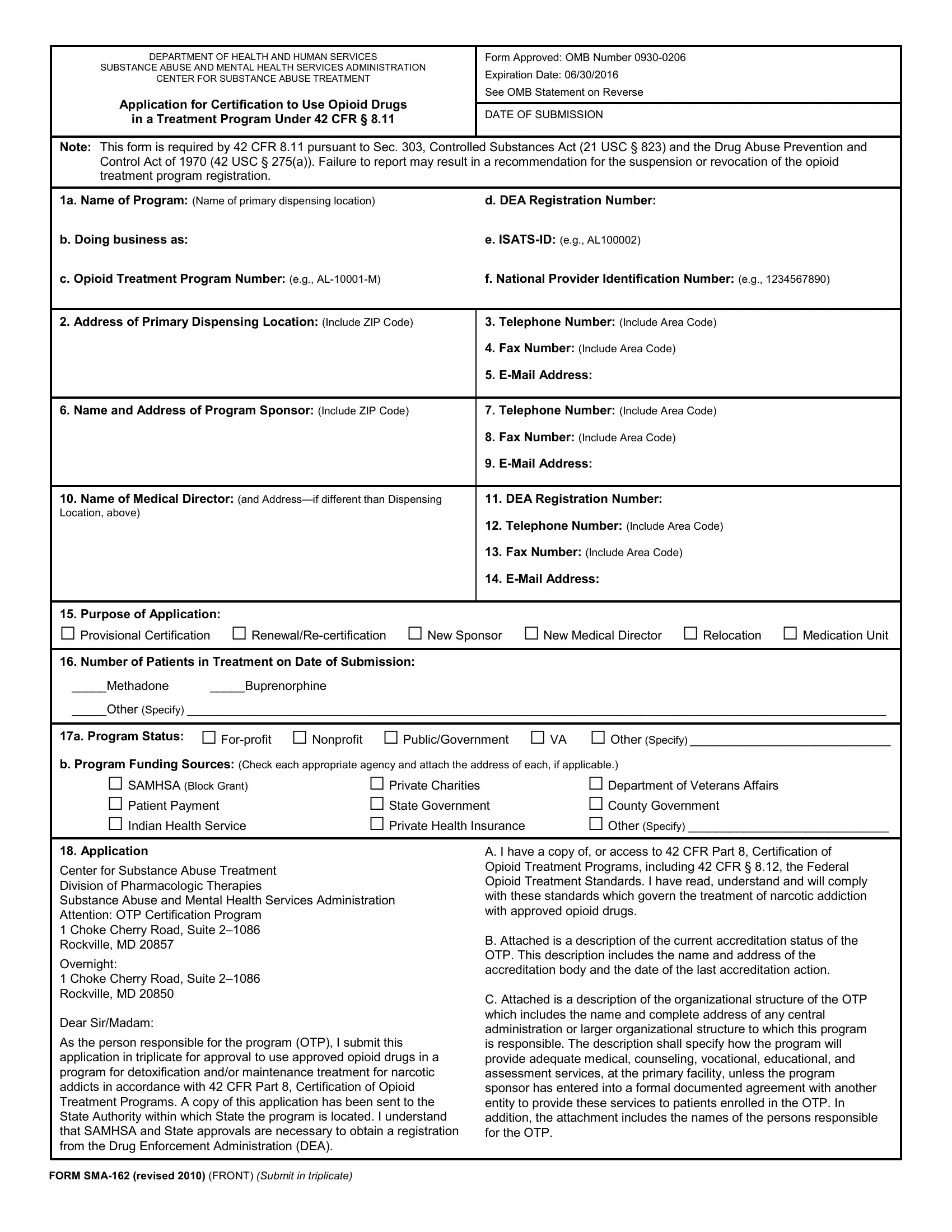
D. Attached are the names, addresses, and a description of each hospital, |
I. I shall comply with the security standards for the distribution of |
institution, clinical laboratory, or other facility used by this program to |
controlled substances, as required by 21 CFR § 1301, Registration of |
provide the necessary medical and rehabilitative services. |
Manufacturers, Distributors, and Dispensers of Controlled Substances. |
E. A medical director will be designated to assume responsibility for |
J. I agree to comply with the conditions of certification set forth under |
administering all medical services performed by the program. If a medical |
42 CFR § 8.11(f). In addition, I shall allow, in accordance with Federal |
director is responsible for more than one program, the feasibility of such |
controlled substance laws and Federal confidentiality laws, inspections |
an arrangement will be documented and submitted to SAMHSA. Within |
and surveys by duly authorized employees of SAMHSA, by |
three weeks of any replacement of the medical director, I shall notify |
accreditation bodies, the DEA, and by authorized employees of any |
SAMHSA. |
relevant State or Federal governmental authority. I agree that OTPs |
|
must operate in accordance with Federal opioid treatment standards |
F. Attached is the address of each medication unit or other facility under |
and accreditation elements. |
control of the OTP. Any new dispensing site for this program, including |
|
|
medication units shall be approved by SAMHSA and the State authority |
K. I agree to adhere to all rules, directives, and procedures set forth in |
prior to its use. SAMHSA and the State authority shall be notified within |
42 CFR Part 8, and any regulation regarding the use of an opioid drug |
three weeks of the deletion of any facility used to dispense opioid |
for the treatment of narcotic addiction which may be promulgated in |
treatment drugs. |
the future. I shall inform other individuals who work in this treatment |
|
program of the provisions of this regulation, and monitor their |
G. A patient records system will be established and maintained to |
activities to assure compliance with the provisions. |
document and monitor patient care in this program. It shall be maintained |
|
|
so as to comply with the Federal and State reporting requirements |
L. I understand that failure to abide by the rules directives, and procedures |
relevant to narcotic treatment. A drug dispensing record will be maintained |
described above may cause a suspension or revocation of approval of my |
to show dates, quantity, and batch or code marks of the drug administered |
registration by the Drug Enforcement Administration. |
or dispensed, traceable to specific patients. This drug dispensing record |
|
|
must be retained for a period of three years from the date of dispensing. |
M. As program sponsor, I certify that the information submitted in |
|
this application is truthful and accurate. |
H. I have a copy of, or access to 42 CFR Part 2, Confidentiality of Alcohol |
|
|
and Drug Abuse Patient Records. I have read and understand the |
|
|
requirements to maintain the confidentiality of alcohol and drug abuse |
|
|
treatment patient records. I agree to protect the identity of all patients in |
|
|
accordance with the regulations. |
|
|
|
|
|
Program Sponsor: (Signature) |
Date: |
|
|
|
Please send three copies of this form and all attachments to: |
Center for Substance Abuse Treatment |
Division of Pharmacologic Therapies |
Substance Abuse and Mental Health Services Administration |
Attention: OTP Certification Program |
1 Choke Cherry Road, Suite 2–1086 |
Rockville, MD 20857 |
|
|
Overnight: |
|
|
1 Choke Cherry Road, Suite 2–1086 |
Rockville, MD 20850 |
|
|
and two copies to the appropriate State authority. |
The preferred method for submitting this form to CSAT/DPT is online at the DPT Web site, http://dpt.samhsa.gov. The Web site contains |
complete instructions for preparing and submitting your request. If you are unable to submit online, the form may be e-mailed as an |
attachment to otp@samhsa.hhs.gov or sent by traditional mail (include three copies of all attachments) to the mailing address above. |
|
|
Paperwork Reduction Act Statement |
Public reporting burden for this collection of information is estimated to average between 6 minutes and 1 hour per response, including the time for |
reviewing instructions, searching existing data sources, gathering and maintaining the data needed, and completing and reviewing the collection of |
information. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing this |
burden to SAMHSA Reports Clearance Officer; Paperwork Reduction Project (0930-0206); Suite 7-1043, 1 Choke Cherry Road, Rockville, MD 20857. |
An agency may not conduct or sponsor, and a person is not required to respond to, a collection of information unless it displays a currently valid OMB |
control number. The OMB control number for this project is 0930-0206. |
|
|
|
|
|
FORM SMA-162 (revised 2010) (BACK) |
|
|