APPLICATION
Sanofi Patient Connection® is a program (the “Program”) to help you get access to the medications and resources you need at no cost. Patient Assistance Connection is part of the Program that provides select Sanofi prescription medications and vaccines, at no cost, if you meet certain eligibility requirements. Patient Assistance Connection is made possible through Sanofi Cares North America.
Who may be eligible for Patient Assistance Connection?
In order to be eligible for this portion of the Program, you must meet the following requirements:
•You must be a resident of the U.S. or the U.S. territories and be under the care of a licensed healthcare provider authorized to prescribe, dispense and administer medicine in the U.S.
•You must have an annual household income of [≤400%] of the current Federal Poverty Level. If you may be eligible for Medicaid, you will be required to provide documentation of Medicaid denial before being assessed for patient assistance eligibility.
•If you are enrolled in Medicare Part D, you may also be eligible based on the income criteria noted above.
•You must have no insurance coverage or, for commercially insured patients, have no access to the prescribed product or treatment via your insurance.
•For Vaccines, you must be 19 years of age or older (except for IMOVAX® Rabies and IMOGAM® Rabies-HT).
How do I apply?
Complete page 2, sign page 3, then bring or send the form to your healthcare provider to complete and sign page 4. Missing information may delay processing of your application. Your completed application may be submitted by your healthcare provider as follows:
U.S. Mail |
Fax |
Secure Provider Portal* |
Sanofi Patient Connection |
1.888.847.1797 |
www.visitspconline.com |
PO Box 222138 |
|
*Excluding Mozobil® and Thymoglobulin® |
Charlotte, NC 28222-2138 |
|
What happens next?
When we receive your application, we will review it to see if you qualify for Patient Assistance Connection. If you are eligible:
1.You and your healthcare provider will receive a letter notifying you of enrollment. If you are a Medicare Part D patient, your plan sponsor will also receive a letter notifying it of your enrollment.
2.You will be enrolled for 12 months. If you are a Medicare Part D patient, you will be enrolled through the end of the calendar year.
3.Your medication will be sent directly to your healthcare provider’s office in approximately 5-7 business days from when you are approved.
If you do not qualify for Patient Assistance Connection, we will send you and your healthcare provider a letter with the reason for denial.
Do not include Patient Medical Records with this application.
|
© 2021 Sanofi US Services, Inc. |
|
P: 1.888.847.4877 · F: 1.888.847.1797 |
|
1 of 5 |
P.O. Box 222138 · Charlotte, NC · 28222-2138 |
|
MAT-US-2109597-v1.0-11/2021 |
|
|
|
APPLICATION
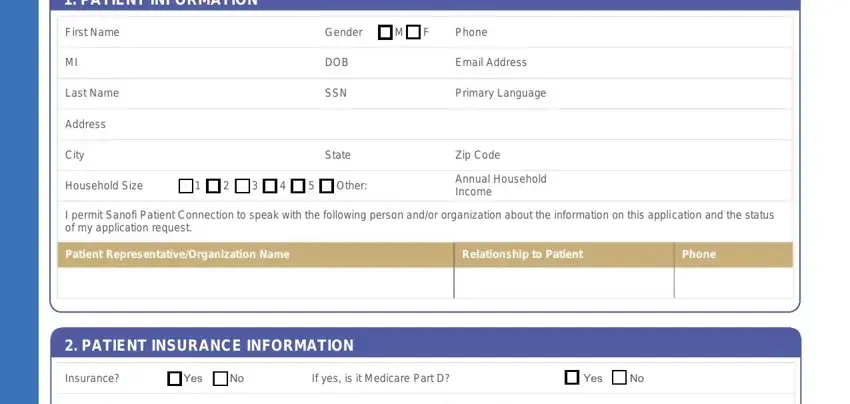
1. PATIENT INFORMATION
First Name |
Gender |
M F |
Phone |
MI |
DOB |
|
Email Address |
Last Name |
SSN |
|
Primary Language |
Address |
|
|
|
City |
State |
|
Zip Code |
Household Size |
1 2 3 4 5 Other: |
|
Annual Household |
|
Income |
|
|
|
I permit Sanofi Patient Connection to speak with the following person and/or organization about the information on this application and the status of my application request.
Patient Representative/Organization Name |
Relationship to Patient |
Phone |
2. PATIENT INSURANCE INFORMATION
Insurance? |
Yes |
No |
If yes, is it Medicare Part D? |
Yes |
No |
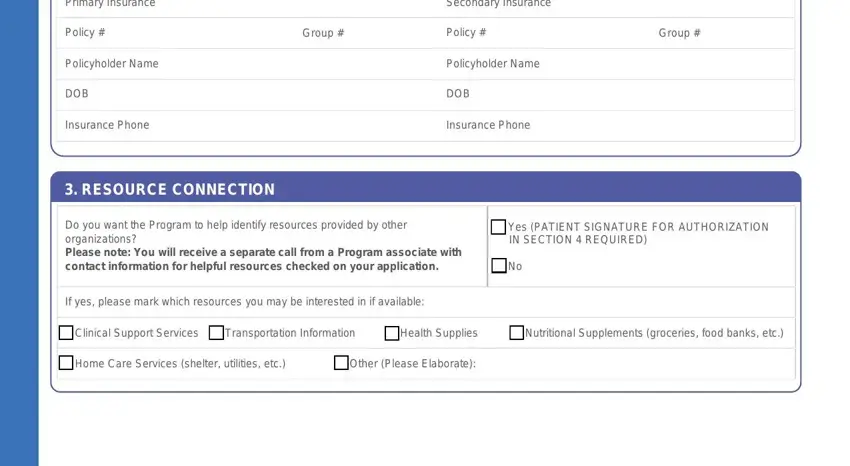
Primary Insurance |
|
|
|
Secondary Insurance |
|
|
Policy # |
|
|
Group # |
Policy # |
|
Group # |
Policyholder Name |
|
|
|
Policyholder Name |
|
|
DOB |
|
|
|
DOB |
|
|
Insurance Phone |
|
|
|
Insurance Phone |
|
|
3. RESOURCE CONNECTION
Do you want the Program to help identify resources provided by other organizations?
Please note: You will receive a separate call from a Program associate with contact information for helpful resources checked on your application.
If yes, please mark which resources you may be interested in if available:
Yes (PATIENT SIGNATURE FOR AUTHORIZATION IN SECTION 4 REQUIRED)
No
|
|
|
|
Clinical Support Services Transportation Information |
Health Supplies |
Nutritional Supplements (groceries, food banks, etc.) |
Home Care Services (shelter, utilities, etc.) |
Other (Please Elaborate): |
|
Do not include Patient Medical Records with this application.
|
© 2021 Sanofi US Services, Inc. |
|
P: 1.888.847.4877 · F: 1.888.847.1797 |
|
2 of 5 |
P.O. Box 222138 · Charlotte, NC · 28222-2138 |
|
MAT-US-2109597-v1.0-11/2021 |
|
|
|
APPLICATION
4. PATIENT AUTHORIZATION (REQUIRED)
Please read the following carefully, then date and sign where indicated below.
Income Verification: Sanofi Patient Connection and its authorized third party agents will use my date of birth or social security number and/or additional demographic information as needed to access my credit information and information derived from public and other sources to estimate my income in conjunction with the eligibility determination process. As a soft credit inquiry, this option will not impact my credit score . Sanofi Patient Connection and its authorized third party agents reserve the right to ask for additional documents and information at any time.
I state that the information and documents provided in connection with this application are complete and accurate. I agree to immediately inform a Program representative and my Doctor/ Healthcare Provider if my income or insurance status changes during the course of my participation in this Program.
HIPAA Consent: I authorize my healthcare providers and staff; my health insurer, health plan or programs that provide me health benefits (together, “Health Insurers”) to disclose to, Sanofi US, its affiliated companies (i.e. Sanofi Pasteur U.S. and Genzyme, a Sanofi Comp any), Sanofi Cares North America, and authorized third party agents involved in administration of this Program, (collectively “Program Sponsor”), health information about me, including information related to my medical condition, treatment, health insurance coverage, claims, prescriptions and referral to and enrollment in this Program for purposes of determining my participation in, and administering, the Program, which may include contacting me as well as my Doctor/Healthcare Provider, office/hospital staff, insurer (public/private) or others. I understand a representative from Sanofi may contact me for follow-up on any adverse event I may report regarding a Sanofi product. I authorize and consent to release of identifiable information about me including medical, financial and insurance records and information as required for participation in the Program. I understand that identifiable information about me will be kept confidential and will not be further used or disclosed except to administer the Program, or as required by law. I understand that information I authorize to be disclosed may be re-disclosed and is no longer protected by Federal privacy regulations. I agree that this authorization is voluntary and that I may refuse to sign this authorization. Refusal to sign will not affect my ability to obtain treatment but I will not be able to participate in this Program. Unless revoked, this authorization shall remain in effect throughout my participation in the Program, including subsequent reapplication as required. I may withdraw this authorization at any time by written notification to my Doctor/Healthcare Provider; however, withdrawal of authorization will terminate my participation in this Program and will not affect information already disclosed under this Authorization.
I understand that it is my responsibility to follow-up with my prescriber or the Program to make sure that my re-orders, as appropriate, are requested in a timely manner by my Provider so I do not run out of medication. I understand that Sanofi US and Sanofi Cares North America reserve the right at any time and without notice to modify or change eligibility criteria or discontinue this Program.
|
Patient Authorization (REQUIRED) |
By signing below, I acknowledge that I have read and agree to the Patient Authorization to |
|
Use and Disclose Health Information above. |
|
|
|
Patient/Representative Signature (REQUIRED) |
|
|
Printed Name |
Date |
5. PATIENT CONSENT
Please read the following carefully, then date and sign where indicated below.
I authorize the Program to contact me by mail, telephone, or e-mail, with information about the Program, disease state and products, promotions, services, and research studies, and to ask my opinion about such information and topics, including market research and disease-related surveys. I further authorize the Program to de-identify my health information and use it in performing research, including linkage with other de-identified information the Program receives from other sources, education, business analytics, marketing studies, or for other commercial purposes. I understand that entities operating or administering parts of the Program may share identifiable health information with one another in order to de-identify it for these purposes and as needed to perform the Services or to send the communications listed above (the “Communications”). I understand and agree that the Program may use my health information for these purposes and may share my health information with my doctors, specialty pharmacies, and insurers. I understand that I may be contacted by the Program in the event that I report an adverse event associated with a Sanofi product.
I understand that I do not have to opt in to receive the Communications, and that I can still receive patient assistance through the Program, as prescribed by my physician. I may opt out of receiving Communications offered by the Program, at any time by notifying a Program representative by telephone at 1-800- 633-1610 or by mailing a letter to Sanofi US Customer Services, P.O. Box 5925 Mailstop 55A-220A5, Bridgewater, NJ 08807-5925. I also understand that the Services may be revised, changed, or terminated at any time.
Patient Consent |
By signing below, I acknowledge that I have read and agree to the Patient Consent above. |
Patient/Representative Signature |
|
Printed Name |
Date |
Do not include Patient Medical Records with this application.
|
© 2021 Sanofi US Services, Inc. |
|
P: 1.888.847.4877 · F: 1.888.847.1797 |
|
3 of 5 |
P.O. Box 222138 · Charlotte, NC · 28222-2138 |
|
MAT-US-2109597-v1.0-11/2021 |
|
|
|
APPLICATION
6. TO BE COMPLETED BY THE HEALTHCARE PROVIDER (HCP)
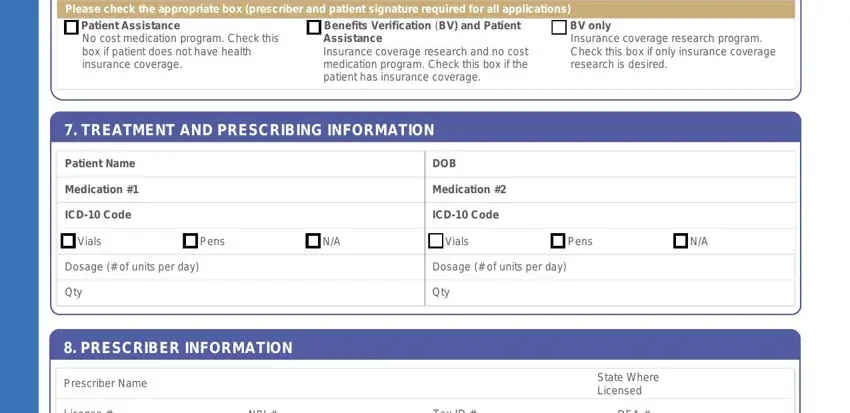
Please check the appropriate box (prescriber and patient signature required for all applications)
Patient Assistance |
Benefits Verification (BV) and Patient |
BV only |
No cost medication program. Check this |
Assistance |
Insurance coverage research program. |
box if patient does not have health |
Insurance coverage research and no cost |
Check this box if only insurance coverage |
insurance coverage. |
medication program. Check this box if the |
research is desired. |
|
patient has insurance coverage. |
|
7. TREATMENT AND PRESCRIBING INFORMATION
Patient Name |
|
|
DOB |
|
|
Medication #1 |
|
|
Medication #2 |
|
|
ICD-10 Code |
|
|
ICD-10 Code |
|
|
Vials |
Pens |
N/A |
Vials |
Pens |
N/A |
Dosage (# of units per day) |
|
Dosage (# of units per day) |
|
Qty |
|
|
Qty |
|
|
8. PRESCRIBER INFORMATION
Prescriber Name |
|
|
State Where |
|
|
Licensed |
|
|
|
License # |
NPI # |
Tax ID # |
DEA # |
Facility Name |
|
|
|
Facility Address* |
|
|
|
City |
|
State |
Zip Code |
Office Contact Name |
|
Title/Role |
|
Primary Phone |
|
Primary Fax |
Primary Email |
*Sanofi product must be shipped to the signing prescriber’s office or hospital address authorized by the prescriber and not to a 3rd party.
I certify that the information provided is current, complete, and accurate to the best of my knowledge. I certify that the Sanofi product is medically necessary for this patient and that I am authorized under State law to prescribe and dispense the requested medication. I certify that I have obtained from my patient all required written authorization for the release of my patient’s personal identification, medical and insurance information to Sanofi US and/or Sanofi Cares North America and their agents and representatives. I understand that any information provided is for the sole use of the Program to verify my patient’s insurance coverage, to assess, if applicable, patient’s eligibility for participation in the Patient Assistance Program and to otherwise administer the Sanofi Patient Connection Program and related services. I understand that I am under no obligation to prescribe any Sanofi product and that I have not received, nor will I receive, any benefit from Sanofi or their agents or representatives for prescribing a Sanofi product. The facility address noted above in Section 8 is my office or hospital address. My signature certifies that any prescription products received from this Program will be used for the above-named patient only and will not be resold nor offered for sale, trade or barter and will not be returned for credit, nor will payment be sought from any payer, patient or other source for product received from the Program.
Prescriber Signature (REQUIRED – no stamps)
SIGN
HERE
Do not include Patient Medical Records with this application.
|
© 2021 Sanofi US Services, Inc. |
|
P: 1.888.847.4877 · F: 1.888.847.1797 |
|
4 of 5 |
P.O. Box 222138 · Charlotte, NC · 28222-2138 |
|
MAT-US-2109597-v1.0-11/2021 |
|
|
|
9. PRODUCT SELECTION
•Adacel® (tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine adsorbed)
•Adlyxin® (lixisenatide) injection
•Admelog® (insulin lispro injection) 100 Units/mL
•Apidra® (insulin glulisine injection) 100 Units/mL
•Imogam® Rabies-HT Immune Globulin, [Human] USP, Heat Treated
•Imovax® Rabies Vaccine [Human Diploid Cell]
•Lantus® (insulin glargine injection) 100 Units/mL
•Lovenox® (enoxaparin sodium injection)*1
•Menactra® Meningococcal (Groups A, C, Y and W-135) Polysaccharide Diptheria Toxoid Conjugate Vaccine
•Mozobil® (plerixafor injection)1
APPLICATION
•Multaq® (dronedarone) Tablets*
•Pentacel® Diptheria and Tetanus Toxoids and Acellular Pertussis Adsorbed, Inactivated Poliovirus and Haemophilus b Conjugate (Tetanus Toxoid Conjugate) Vaccine
•Priftin® (rifapentine) Tablets
•Soliqua® 100/33 (insulin glargine & lixisenatide) injection 100 Units/mL and 33 mcg/mL
•Tenivac® (tetanus and diphtheria toxoids adsorbed)
•Thymoglobulin® [Anti-Thymocyte Globulin (Rabbit)]*,1
•Toujeo® (insulin glargine injection) 300 Units/mL (1.5 mL or 3.0 mL pens)**
*Please see full U.S. prescribing information, including Black Box warning.
**Regular SoloStar® is packaged as 3 pens per pack 450 units/pen; dials up to 80 units per single injection. Max SoloStar® is packaged as 2 pens per pack 900 units/pen; dials up to 160 units per single injection; Max pen dials in 2-unit increments.
1If applying for Drug Replacement (Lovenox®, Mozobil®, and Thymoglobulin®), a copy of the claim, denial, flow sheet(s) and drug dispensing log (with patient name, date of service, product NDC/Lot #, total dosage) must be submitted.
Full U.S. prescribing information for all Sanofi Patient Connection supported products can be accessed at www.visitspconline.com. Sanofi Patient Connection will provide assistance for any medically appropriate use as described in the prescribing information.
10. WHAT DOES A SUCCESSFUL PATIENT ASSISTANCE CONNECTION APPLICATION LOOK LIKE?
To apply for Patient Assistance Connection all information must be complete and include the following:
Patient Information:
•Complete all relevant information on page 2, and sign and date the Patient Authorization on page 3 (REQUIRED).
Healthcare Provider:
•Ask your Healthcare Provider (HCP) to complete page 4 and sign and date it.
•Ask your HCP to mail, fax, or submit through the Provider Portal your completed application.
Missing information may delay processing of application.
Do not include Patient Medical Records with this application.
11. ADDITIONAL INFORMATION
•Sanofi Patient Connection ships most medications in a 90-day supply.
•A representative from Sanofi may contact you for follow-up on any adverse event you may report regarding a Sanofi product.
12. FORM SUBMISSION OPTIONS
U.S. Mail |
Fax |
Sanofi Patient Connection |
1.888.847.1797 |
PO Box 222138 |
|
Charlotte, NC 28222-2138 |
|
Do not include Patient Medical Records with this application.
|
© 2021 Sanofi US Services, Inc. |
5 of 5 |
|
MAT-US-2109597-v1.0-11/2021 |
|
|
Secure Provider Portal*
www.visitspconline.com
*Excluding Mozobil® and Thymoglobulin®
P:1.888.847.4877 · F: 1.888.847.1797 P.O. Box 222138 · Charlotte, NC · 28222-2138