The flu vaccine is one of the most important vaccines you can get to protect yourself and your loved ones from the risk of serious illness. However, it's important to keep track of your vaccinations and keep a record form to ensure you receive all the necessary doses. This blog post will provide you with a template for a flu vaccine record form that you can use to keep track of your vaccinations. It will also provide information on when to get vaccinated and how to stay healthy during flu season. Stay safe this winter and get vaccinated against the flu!
| Question | Answer |
|---|---|
| Form Name | Flu Vaccine Record Form |
| Form Length | 2 pages |
| Fillable? | No |
| Fillable fields | 0 |
| Avg. time to fill out | 30 sec |
| Other names | flu vaccination card template, influenza vaccine card, flu vaccine log sheet, flu vaccine record card |
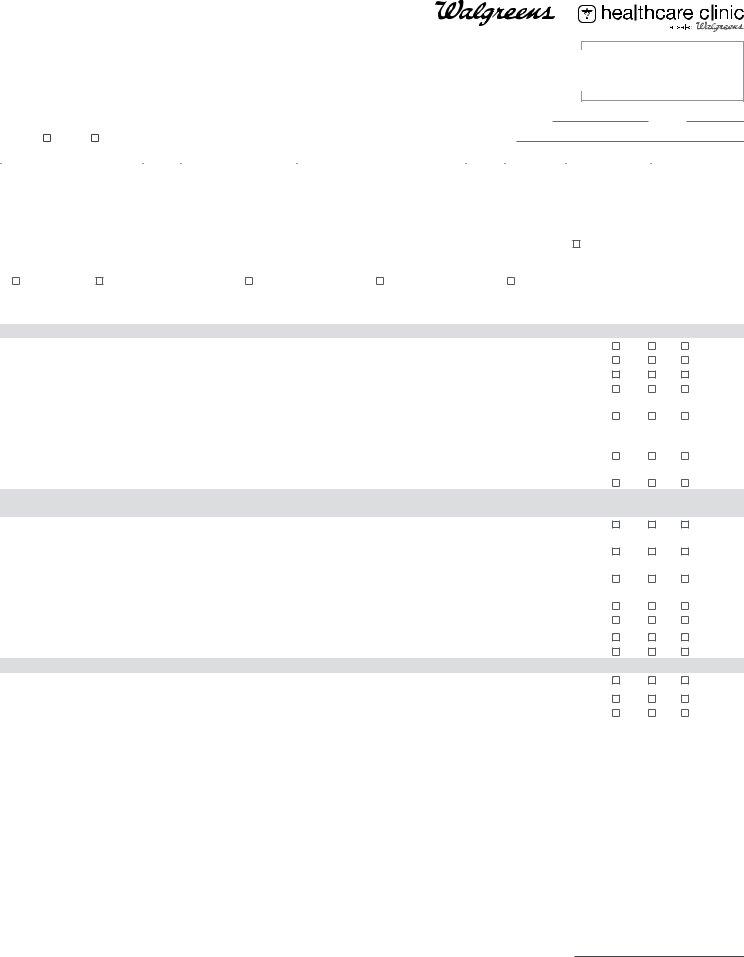
Vaccine Administration Record (VAR) Informed Consent for Vaccination for all healthcare providers*
†
PATIENT: COMPLETE SECTIONS A, B, C
PROVIDER: COMPLETE SECTION D (reverse side)
SECTION A (Please print clearly.)
First name: |
|
|
|
Last name: |
|
|
Date of birth: |
Gender: Female |
Male Home phone: |
|
|
|
Mobile phone: |
||
Race (select one or more)
Native American or Alaska Native Asian Black or
STAMP
STORE
Age:
Ethnicity (select one)
Hispanic or Latino Not Hispanic or Latino
Home address: |
|
|
|
|
|
|
|
|
City: |
|
|
|
|
State: |
|
|
|
|
|
ZIP code: |
|
|
||||||
Email address: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
Doctor/primary care provider name: |
|
|
|
|
|
|
|
Phone number: |
|
|
|
|
|
|||||||||||||||
Address: |
|
|
|
|
|
|
|
City: |
|
|
|
State: |
|
|
I do not have a primary care doctor/provider |
|||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
I want to receive the following immunization(s): |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
Flu (influenza) |
Pneumonia (pneumococcal) |
Shingles (herpes zoster) |
Tdap (whooping cough) |
Other: |
|
|
|
|
|
|
|
|
|
|
|
||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
The following questions will help us determine your eligibility to be vaccinated today. For all vaccines: Please answer questions |
|
|
|
|||||||||||||||||||||
SECTION B |
|
|
|
|
||||||||||||||||||||||||
|
For live vaccines (e.g., MMR or shingles): Please answer questions |
|
|
|
|
|||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
All vaccines |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
1. |
Are you currently sick with a moderate to high fever, vomiting/diarrhea? |
|
|
|
|
|
|
|
|
|
|
|
|
|
Yes |
No |
Don’t know |
|||||||||||
2. |
Have you ever fainted or felt dizzy after receiving an immunization? |
|
|
|
|
|
|
|
|
|
|
|
|
|
Yes |
No |
Don’t know |
|||||||||||
3. |
Have you ever had a reaction after receiving an immunization? |
|
|
|
|
|
|
|
|
|
|
|
|
|
Yes |
No |
Don’t know |
|||||||||||
4. |
Do you have an immunocompromising condition (e.g., cancer, leukemia, lymphoma, HIV/AIDS, transplant), functional, |
|
|
|
|
Yes |
No |
Don’t know |
||||||||||||||||||||
|
or anatomic asplenia, CSF leak or cochlear implant? |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||
5. |
Do you have allergies to latex, medications, food or vaccines? (Examples: eggs, bovine protein, gelatin, gentamicin, polymyxin, |
Yes |
No |
Don’t know |
||||||||||||||||||||||||
|
neomycin, phenol, yeast or thimerosal) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
a. If yes, please list: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
6. |
Have you ever had a seizure disorder for which you are on seizure medications, a brain disorder, |
Yes |
No |
Don’t know |
||||||||||||||||||||||||
|
other nervous system problems? |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
7. |
FOR WOMEN: Are you pregnant or considering becoming pregnant in the next month? |
|
|
|
|
|
|
|
|
|
|
Yes |
No |
Don’t know |
||||||||||||||
Live vaccines (Chicken pox, flu nasal spray, MMR, oral typhoid, shingles, Yellow fever) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
Only answer these questions if you are receiving any immunization listed above. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||
8. |
Are you currently on home infusions, weekly injections (such as adalimumab, infliximab and etanercept), |
|
|
|
|
Yes |
No |
Don’t know |
||||||||||||||||||||
|
methotrexate, azathioprine or |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
9. |
Have you received any vaccinations or skin tests in the past four weeks? |
|
|
|
|
|
|
|
|
|
|
|
|
|
Yes |
No |
Don’t know |
|||||||||||
|
a. If yes, please list: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
10. |
Have you received a transfusion of blood, blood products or been given a medication called immune (gamma) globulin |
|
|
|
|
Yes |
No |
Don’t know |
||||||||||||||||||||
|
in the past year? |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
11. |
Are you currently taking |
|
|
|
|
Yes |
No |
Don’t know |
||||||||||||||||||||
12 |
Do you have a history of thymus disease (including myasthenia gravis), thymoma or prior thymectomy? (Yellow fever only) |
|
|
|
|
Yes |
No |
Don’t know |
||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
13. |
Are you currently taking any antibiotics or antimalarial medications? (Oral typhoid only) |
|
|
|
|
|
|
|
|
|
|
Yes |
No |
Don’t know |
||||||||||||||
14. |
Do you have a history of thrombocytopenia or thrombocytopenic purpura? (MMR only) |
|
|
|
|
|
|
|
|
|
|
Yes |
No |
Don’t know |
||||||||||||||
Flu nasal spray (FluMist® Quadrivalent) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
15. |
For patients 18 years of age and younger only: Are you receiving aspirin therapy or |
|
|
|
|
Yes |
No |
Don’t know |
||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
16. |
For patients 5 years of age and younger only: Is there a history of asthma or wheezing? |
|
|
|
|
|
|
|
|
|
|
Yes |
No |
Don’t know |
||||||||||||||
17. |
Do you have a nasal condition serious enough to make breathing difficult, such as a very stuffy nose? |
|
|
|
|
|
|
|
|
|
|
Yes |
No |
Don’t know |
||||||||||||||
SECTION C
I certify that I am: (a) the patient and at least 18 years of age; (b) the parent or legal guardian of the minor patient; or (c) the legal guardian of the patient. Further, I hereby give my consent to the healthcare provider of Walgreens or Take Care Health ServicesSM, as applicable, to administer the vaccine(s) I have requested above. I understand that it is not possible to predict all possible side effects or complications associated with receiving vaccine(s). I understand the risks and benefits associated with the above vaccine(s) and have received, read and/or had explained to me the Vaccine Information Statements on the vaccine(s) I have elected to receive. I also acknowledge that I have had a chance to ask questions and that such questions were answered to my satisfaction. Further, I acknowledge that I have been advised to remain near the vaccination location for approximately 15 minutes after administration for observation by the administering healthcare provider. On behalf of myself, my heirs and personal representatives, I hereby release and hold harmless Walgreens or Take Care Health ServicesSM, as applicable, its staff, agents, successors, divisions, affiliates, subsidiaries, officers, directors, contractors and employees from any and all liabilities or claims whether known or unknown arising out of, in connection with, or in any way related to the administration of the vaccine(s) listed above. I acknowledge that: (a) I understand the purposes/benefits of my state’s immunization registry (“State Registry”) and my state’s health information exchange (“State HIE”); and (b) Walgreens or Take Care Health ServicesSM, as applicable, may disclose my immunization information to the State Registry, to the State HIE, or through the State HIE, to the State Registry, for purposes of public health reporting
or to my health care providers enrolled in the State Registry and/or State HIE for purposes of care coordination. I acknowledge that, depending upon my state’s law, I may prevent, by using a
Signature: |
|
Date: |
(Parent or guardian, if minor)
*Healthcare providers can be an
†Patient care services at Healthcare Clinic at select Walgreens provided by Take Care Health Services, an independently owned professional corporation whose licensed healthcare professionals are not employed by or agents of Walgreen Co. or its subsidiaries, including Take Care Health Systems, LLC. Walgreen Co. and its subsidiary companies provide management services to provider practices,
14IM0007
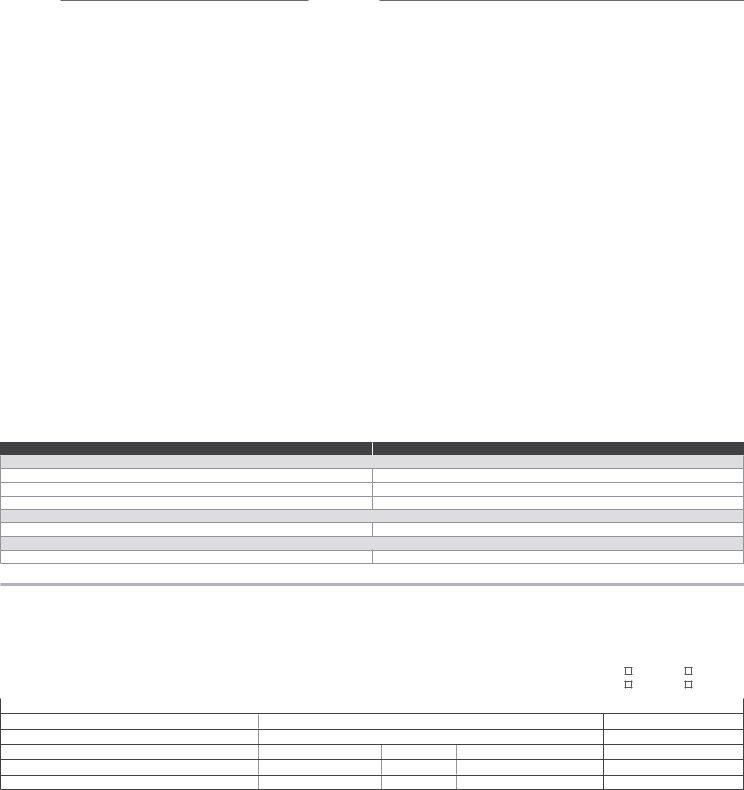
First name:Last name:
SECTION D |
|
|
|
HEALTHCARE PROVIDER ONLY |
|
|
Complete BEFORE vaccine administration |
|
|
|
|||
|
|
|
|
|
||
Vaccine |
Route |
Dosage |
Lot # |
Expiration date |
||
Influenza |
intramuscular |
0.25mL: |
|
|
||
0.5mL: >36 months |
|
|
||||
|
|
|
|
|
|
|
Influenza (intradermal) |
intradermal |
0.1mL (prefilled) |
|
|
||
|
|
|
|
|
||
Influenza (nasal) |
intranasal |
0.1mL each nostril |
|
|
||
Influenza (high dose) |
intramuscular |
0.5mL (prefilled) |
|
|
||
|
|
|
|
|
||
Chicken pox (varicella) |
subcutaneous |
0.5mL |
|
|
||
|
|
|
|
|
|
|
Hepatitis A |
intramuscular |
1mL: Adults ≥19 years |
|
|
||
0.5mL: Adolescents ≤ 18 years |
|
|
||||
|
|
|
|
|
|
|
Hepatitis B |
intramuscular |
1mL: Adults ≥20 years |
|
|
||
0.5mL: Adolescents ≤ 19 years |
|
|
||||
|
|
|
|
|
|
|
Hepatitis A/B (Twinrix®) |
intramuscular |
1mL: Adults ≥18 years |
|
|
||
Human papillomavirus |
intramuscular |
0.5mL |
|
|
||
Japanese encephalitis |
intramuscular |
0.5mL |
|
|
||
|
|
|
intramuscular |
|
|
|
Meningococcal (meningitis) |
(subcutaneous – |
0.5mL |
|
|
||
|
|
|
Menomune® only) |
|
|
|
MMR (measles, mumps, rubella) |
subcutaneous |
0.5mL |
|
|
||
Pneumococcal (Pneumovax®) |
intramuscular |
0.5mL |
|
|
||
Pneumococcal (Prevnar®) |
intramuscular |
0.5mL (prefilled) |
|
|
||
Polio |
intramuscular |
0.5mL |
|
|
||
Rabies |
intramuscular |
1mL |
|
|
||
Shingles (herpes zoster) |
subcutaneous |
0.65mL |
|
|
||
Td (tetanus and diphtheria) |
intramuscular |
0.5mL |
|
|
||
Tdap (tetanus, diphtheria |
intramuscular |
0.5mL |
|
|
||
and pertussis) |
|
|
||||
|
|
|
|
|||
Typhoid (live oral) |
orally |
1 capsule by mouth every other |
|
|
||
day until all taken |
|
|
||||
|
|
|
|
|
|
|
Typhoid (inactive injectable) |
intramuscular |
0.5mL |
|
|
||
Yellow fever |
subcutaneous |
0.5mL |
|
|
||
|
|
|
|
|||
Needle size |
|
Patient gender/weight |
|
|||
Intramuscular injection is in the deltoid |
|
|
|
|||
‡ to 1 inch needle
1 to 1½ inch needle
1½ inch needle
Female or male weighing less than 130 lbs
Female
Female 200+ lbs; male 260+ lbs
Subcutaneous injection is in the upper arm (posterolateral)
inch needle
All patients
Intradermal injection is in the deltoid
Prefilled syringe
All patients
‡A 5/8 inch needle may be used for patients weighing less than 130 lbs (<60kg) for IM injection in the deltoid muscle only if the subcutaneous tissue is not bunched and the injection is made at a
I have verified the immunization(s) that the patient requested meets state, age and vaccine restrictions. |
Initial here: |
|
|
|
|
I have verified the requested immunization is the same as the product prepared. |
Initial here: |
|
|
|
|
I have verified the expiration date of the product is greater than today’s date. |
Initial here: |
|
|
|
|
For Zostavax®, MMR II®, Varivax®, |
|
|
insert’s instructions. |
Initial here: |
|
|
|
|
For patients younger than 9 years of age requesting the influenza vaccine: |
|
|
Did you verify if a second dose is needed? |
Yes |
No |
If this is the second dose, have 28 days elapsed since the first dose? |
Yes |
No |
|
|
|
Complete AFTER vaccine administration |
|
|
|
|
|
Vaccine
NDC |
Dosage |
Site of administration (circle site) VIS published date |
|
|
L /R IM/SQ |
Immunizer name (print): |
|
|
Immunizer signature: |
|
|
|
|
|
|
Title: |
|
|
||||||
If applicable, intern name (print): |
|
|
|
Administration date: |
|
|
|
Date VIS given to patient: |
|
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Immunization billing notes section (complete all applicable fields) |
|
|
|
|
|
|||||||||||||
Insurance name: |
|
|
|
|
|
|
|
Payer ID/BIN: |
|
|
|
|
|
|
|
|||
Cardholder name: |
|
|
Recipient ID: |
|
|
Group ID: |
|
|||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Notes |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
14IM0007